C2H2 Hybridization
C2H2 Hybridization
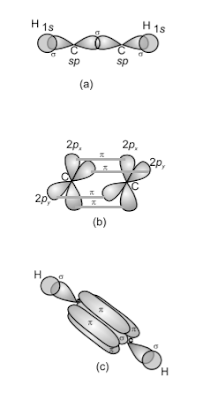
In the
formation of ethyne molecule, both the carbon atoms undergo sp-hybridization
having two unhybridized orbital i.e., 2py and 2px.
One sp hybrid orbital of one carbon
atom overlaps axially with sp hybrid orbital of the other carbon atoms
to form C-C sigma bonds. While the other hybridized orbital of each carbon atom
overlaps axially with the half filled s orbital of hydrogen atoms
forming bonds. Each of the two unhybridised
p orbitals of both the carbon atoms overlaps sidewise to form two 𝜋 bonds between the carbon atoms. so the triple
bonds between the two carbon atoms is made up of one sigma and two pi bonds as
shown in the below figure.
Shouldn't there be 4 unhybridized orbitals in one molecule of ethane 2 from each carbon?
ReplyDelete