Aldehydes
Aldehydes
Aldehydes have the general formula CnH2nO
and it contain the oxo (carbonyl) group. In aldehydes, the functional group is
–CHO, i.e., one of the available valencies of the carbonyl group is attached to
hydrogen, and so the aldehyde group occurs at the end of a chain.
Nomenclature
The lower members are commonly named after the acids that
they form on oxidation. The suffix of the names of acids is –ic (the
names of the trivial system are used); this suffix is deleted and replaced by
aldehyde, e.g.,
HCHO (O)⟶ HCO2H
Formaldehyde formic acid
(CH3)2CHCHO (O)⟶ (CH3)2CHCO2H
Isobutyraldehyde isobutyric acid
The positions of side-chains or substituents are indicated
by Greek letters, the α carbon atom being the one adjacent to the
aldehyde group, e.g.,
According to the I.U.P.A.C. system of nomenclature,
aldehydes are designated by the suffix –al, which is added to the name
of the hydrocarbon from which they are derived. The longest carbon chain
containing the aldehyde group is chosen as the parent hydrocarbon; the
positions of side-chains or substituents are indicated by numbers, and the
aldehyde group is given the number 1, which may be omitted from the name if it
is the only functional group present in the compounds e.g.,
CH3CHO ethanol
CH3CH2CHCH(CH3)CH2CH3 2-ethyl-3-methylpentanal
↓
CHO
General methods of preparation of aldehydes
1. By the oxidation or dehydrogenation of a primary alcohol
t-Butyl
chromate (Prepared by adding chromium trioxide to t-butanol)
oxidises primary alcohols to
aldehydes almost quantitatively
(Oppenaure et al., 1949).
2. By heating a mixture of the calcium salts of formic acid
and any one of its homologues (yield: variable due to many side reactions):
(RCO2)2Ca +
(HCO2)Ca ⟶ 2RCHO
+ 2CaCO3
3. By passing a mixture of the vapors of formic acid and any
one of its homologues over manganous oxide as catalyst at 300oC:
RCO2H
+ HCO2H (MnO) ⟶ RCHO + CO2 + H2O
R2CO and RCHO are obtained as by-products, and
the reaction probably proceeds via th manganous salt.
4. By the reduction of an acid chloride with hydrogen in
boiling xylene using a palladium catalyst supported on barium sulphate (Rosenmund’s
reduction)
RCOCl + H2 ⟶ RCHO + HCl
Aldehydes are more readily reduced than are acid chlorides,
and therefore one would expect to obtain the alcohol as the final products. It
is the barium sulphate that prevents the aldehyde from being reduced, acting as
a poison (to the palladium catalyst) in this reaction. Generally, when the
Rosenmund reduction is carried out, a small amount of quinoline and sulphur is
added; these are very effective in poisoning the catalyst in the aldehyde
reduction.
5. By means of a Grignard reagent and formic ester.
General properties of aldehydes
Dipole moment
measurements of aldehydes has shown that the values are larger than can be
accounted for by the inductive effect of the oxygen atom, but can be accounted
for if carbonyl compounds are resonance hybrids:
Thus the carbon atom has a positive charge and consequently
can be attached by nucleophilic reagents. The carbonyl group also exhibits
basic properties; it is readily protonated by strong acids to form oxonium
salts, since oxygen is more electronegative than carbon, the second resonating
structure will make a larger contribution than the first.
Hence, protonation increases the electrophilic character of
the carbonyl group and so it can be expected that nucleophilic additions will
be catalysed by acids. It should also be noted that, because of the positive
charge on the carbon atom, the CO group has a strong –I effect. Many addition
reactions of carbonyl compounds may be represented by the general equation.
Reaction of aldehydes
1. Catalytic hydrogenation readily converts aldehydes into
primary and secondary alcohols, respectively. Reduction with dissolving metals
also produces alcohols.
This reaction, however may also lead to the formation of a
1,2-glycol.
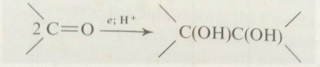
2. Aldehydes add on sodium hydrogen sulphite to form
bisulphate compounds:
These bisulphate compounds are hydroxysulphonic acid salts,
since the sulphur atom is directly attached to the carbon atom. This structure
is supported by work with isotope 34S.
Bisulphate compounds are usually crystalline solids,
insoluble in sodium hydrogen sulphite solution. Since they regenerated the
carbonyl compound when heated with dilute acid or sodium carbonate solution,
their formation affords a convenient means of separating carbonyl compounds
from non-carbonyl compounds.
3. Aldehydes add on hydrogen cyanide to form cyanohydrins. The
carbonyl compound is treated with sodium cyanide and dilute sulphuric acid:
4 aldehydes add on a molecule of a Grignard reagent, and the
complex formed when decomposed with acid, gives a secondary alcohol from an
aldehyde (except formaldehyde, which gives a primary alcohol), and a tertiary
alcohol from a ketone.






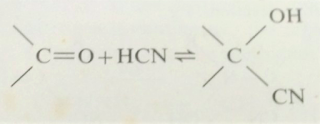

Comments
Post a Comment