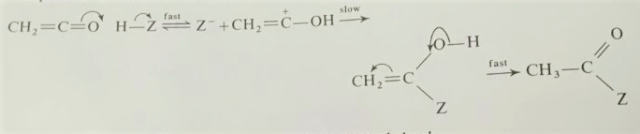
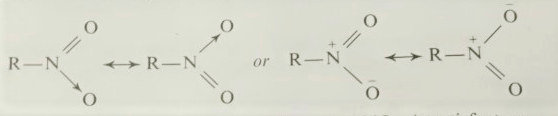Nitrosoalkanes

Nitrosoalkanes The nitrosoalkanes contain a nitroso-group, ーN=O, directly attached to a carbon atom. They are named as the nitroso-derivatives of the corresponding alkanes, e.g., (CH 3 ) 3 CNO 2-methyl-2nitrosopropane General methods of preparation 1. By the addition of nitrosyl chloride or bromide to alkenes, whereby alkene nitrosohalides are formed: CH 2 =CH 2 + NOCl ⟶ ClCH 2 CH 2 NO (2 mol) ⟶ (ClCH 2 CH 2 NO) 2 2. By the action of nitrous acid on certain types of compounds, e.g., secondary nitroalkanes. 3. By the oxidation of primary amines containing a tertiary alkyl group with e.g., Caro’s acid (peroxy-(mono) sulphuric acid): R 3 CNH 2 + 2[O] (H 2 SO 4 ) ⟶ R 3 CNO + H 2 O On the other hand, Emmons (1957) has prepared primary, secondary and tertiary nitroso-compounds by oxidation of amines with neutralized peracetic acid in methylene dichloride (yield: 33-80 per cent). N-Substituted hydrox