Formic Acid : Preparation, Reaction, uses
Formic Acid
Formic acid is also known as methanoic acid. Formic acid is
a pungent corrosive liquid, m.p. 8.4oC, b.p. 100.5oC,
miscible in all proportions with water, ethanol and ether. It forms salts
which, except for the lead and silver salts, are readily soluble in water. Formic
acid is a stronger acid than any of its homologues.
Preparation of Formic acid
Formic acid is prepared industrially by heating sodium
hydroxide with carbon monoxide at 210oC, and at a pressure of 6-10
atmospheres:
NaOH + CI ⟶ HCO2Na
An aqueous solution of formic acid is obtained by distilling
the sodium salt with dilute sulphuric acid:
2HCO2Na
+ H2SO4 ⟶ 2HCO2H + Na2SO4
Anhydrous formic acid is obtained from the aqueous solution
(70-77 per cent) by the addition of butyl formate followed by distillation. The
first fraction is an azeotrope of ester and water, and then the excess of ester
is removed from the formic acid by fractionation.
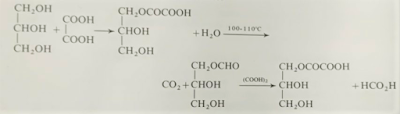
The most convenient laboratory preparation of formic acid is
to heat glycerol with oxalic acid at 100-110oC. Glyceryl monoxalate
is produced, and decomposes into glyceryl monoformate (monoformin) and carbon
dioxide. W hen the evolution of carbon dioxide ceases, more oxalic acid is
added, whereupon formic acid is produced:
The distillate contains formic acid and water. The aqueous
formic acid solution cannot be fractionated to give anhydrous formic acid
because the boiling point of the acid is 100.5oC. the procedure
adopted is to neutralize the aqueous acid solution with lead carbonate, and
concentrated the solution until lead formate crystallizes out. The precipitate
is then recrystallised, dried, and heated at 100oC in a current of
hydrogen sulphide:
(HCO2)2Pb + H2S ⟶ 2HCO2H + PbS
The anhydrous formic acid which distils over contains a small
amount of hydrogen sulphide, and may be free from the latter by adding some dry
lead formate and redistilling. This procedure for obtaining the anhydrous acid
from its aqueous solution can only be used for volatile acids.
Formic acid is dehydrated to carbon monoxide by concentrated
sulphuric acid and when heated under pressure at 160oC, is
decomposed into carbon dioxide and hydrogen.
CO + H2O (H2SO4) ⟵ HCO2H (Heat) ⟶ CO2 + H2
The same decomposition takes place at room temperature in
the presence of catalyst such as iridium, rhodium, etc.
When metallic formats are heated with an alkali, hydrogen is
evolved.
HCO2Na
+ NaOH ⟶ H2 + Na2CO3
When calcium or zinc formate is strongly heated,
formaldehyde is produced:
(HCO2)2Ca ⟶ HCHO + CaCO3
When sodium or potassium formate is rapidly heated to 360oC,
hydrogen is evolved and the oxalate is formed:
2HCO2Na ⟶ (COONa)2 + H2
Formic acid forms esters, but since it is a relatively
strong acid it is not necessary to use a catalyst; refluxing 90 per cent formic
acid with the alcohol is usually sufficient.
Formic acid differs from the rest of the members of the
monocarboxylic acid series in being a powerful reducing agent; it reduces
ammoniacal silver nitrate and the salts of many of the heavy metals, e.g., it
converts mercuric chloride into mercurous chloride.
Comments
Post a Comment